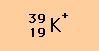
The number of sub-atomic particles (protons, neutrons and electrons) in an atom can be conveniently specified using notation like that shown below for the potassium cation.
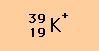
The left subscript (19) is often omitted since the same information is given by the chemical symbol of the element, namely, the number of protons the atom contains and this can always be looked up in a Periodic Table.
The right superscript is omitted if the species has no charge (is neutral).