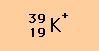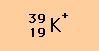Example
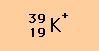
From the symbol above it is possible to deduce that this particular
potassium ion contains
- 19 protons (as do all potassium atoms and ions)
- 20 neutrons ( from mass number [39] - atomic number [19] = number of
neutrons [20])
- 18 electrons (the normal 19 electrons of a potassium atom minus one
because the species has a positive charge, that is, has lost one electron).
Module Index | Topic
Index | Previous Page | Next
Page