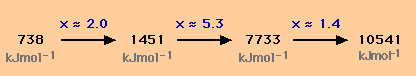
The diagram below shows the first four successive ionisation energies of the element magnesium. Magnesium is in group II of the Periodic Table and has two electrons in its outermost shell.
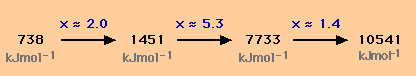
The blue figures above the arrows indicate the approximate factor by which the successive ionisation energies increase, for example
By inspection one can see that there is a large jump in size between the second ionisation energy (1451 kJmol-1) and the third ionisation energy (7733 kJmol-1). This suggests that the third electron is being removed from a different shell to the second electron and that therefore magnesium has two electrons in its outermost shell.
Faced with a question of the form "use the following numerical data for element X to deduce which group of the Periodic Table X comes from", you are strongly advised to calculate the approximate factors by which the successive ionisation energies decrease as the first step in your answer.