The information in an nmr spectrum can consist of any or all of the following items
Chemical shift depends on the environment in which protons find themselves.
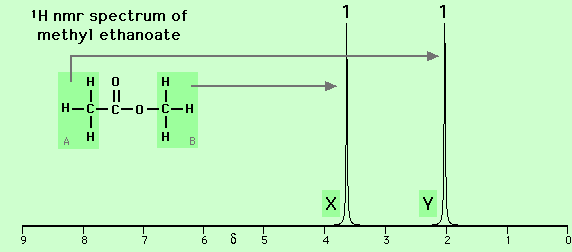
For example, in the nmr spectrum of methyl ethanoate (CH3-CO-O-CH3, see below), the methyl group directly attached to the oxygen atom (-O-CH3) (labelled B and causing the peak labelled X) is in an environment that gives it a higher chemical shift (delta = 3.7 ppm) than the methyl group attached to the carbonyl group (CH3-CO-) (labelled A and causing the peak labelled Y, delta = 2.0 ppm)). The difference in the environments is due largely to the high electronegativity of oxygen compared to hydrogen or carbon.

Relative intensity depends on the number of protons in a given environment.
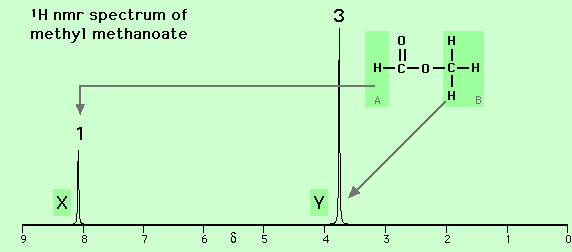
For example, in the nmr spectrum of methyl methanoate (H-CO-O-CH3, see below), the peaks labelled X and Y have a relative intensity of 1 to 3 reflecting the relative number of protons in the two environments within the molecule, that is, one proton near the carbonyl group (H-CO-) (labelled A and causing the peak labelled X, delta = 8.1 ppm) and three protons on the other side of the ester linkage (-O-CH3) (labelled B and causing the peak labelled Y, delta = 3.8 ppm).
The splitting pattern depends on the number of neighbours adjacent to a given proton.
For example, in the nmr spectrum of methyl propanoate (CH3-CH2-CO-O-CH3, see below), the features labelled Y and Z are split into a quartet and a triplet respectively whilst the feature labelled X is present as a singlet only.
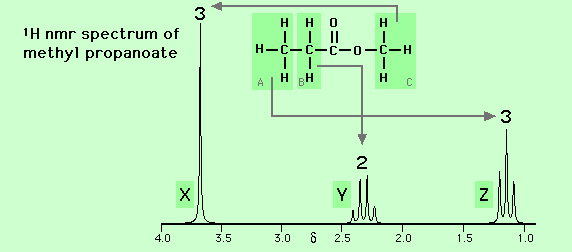
X is due to the methyl group (-CH3, labelled C) which is not affected by spin-spin coupling.
Y is due to the methylene group (-CH2-, labelled B) which is split into a quartet by the influence of the three protons in the neighbouring methyl group (-CH3, labelled A).
Z is due to the methyl group (-CH3, labelled A) which is split into a triplet by the influence of the two protons in the neighbouring methylene group (-CH2-, labelled B).
Note the relative intensities of the features X, Y and Z (3 to 2 to 3) reflecting the number of protons in each of the three environments within the molecule.
Also note the chemical shifts of the three features