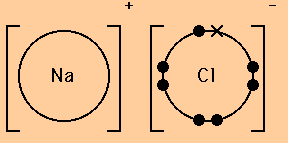
A dot and cross diagram for an ionic compound shows the number of electrons present in the outer shell of the atoms from which the compound was made.
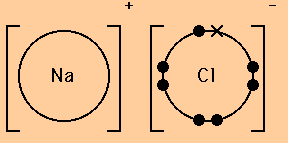
For example, the dot and cross diagram for sodium chloride shows that the outer shell of the sodium (E.C.(Na) = 2, 8, 1) is empty when it has been converted to a sodium ion (E.C.(Na+) = 2, 8). It also shows that the outer shell of the chlorine (E.C.(Cl) = 2, 8, 7) contains eight electrons when it has been converted into a choride ion (E.C.(Cl-) = 2, 8, 8).
Here are two more examples of dot and cross diagrams for ionic compounds.
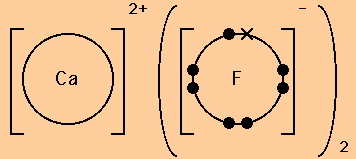
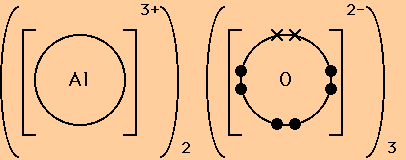
Note the use of round brackets to indicate when 2 or 3 ions of one type are needed to balance the charges and form a neutral compound.
Also note the use of dots to indicate the number of electrons that were originally present in the outer shell of the non-metal atom and crosses to indicate the number of electrons that have been transferred from the metal atom (or atoms) to fill the outer shell of the non-metal.