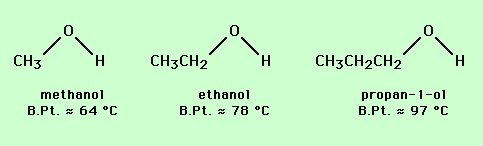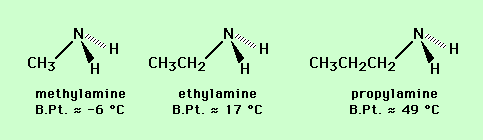
Volatility is a measure of the ease with which a substances melts and/or boils.
Volatile substances melt and boil easily, that is, they melt and boil at comparatively low temperatures.
The opposite of volatile is involatile. An involatile substance melts and boils with difficulty, that is, it melts and boils at a high temperature.
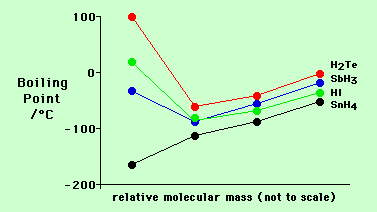
The following graph shows how the volatility of the hydrides of the group 4, 5, 6 and 7 elements varies.

Note the strong influence of hydrogen bonding which explains the low volatility (high boiling point) of HF, H2O and NH3.
Also note that volatility generally decreases (B.Pt. increases) down a group (e.g. CH4, SiH4, GeH4 etc) as the polarisibility of the molecules increases and their temporary dipoles get larger leading to greater temporary dipole/temporary dipoles interactions (van der Waals' forces).
Similar arguments can be used to predict the relative volatility of sets of compounds with similar structures, such as alcohols and amines (see below).