Interpreting graphs of successive ionisation energies
The graph below shows the successive ionisation energies of the element
chlorine (atomic number 17).
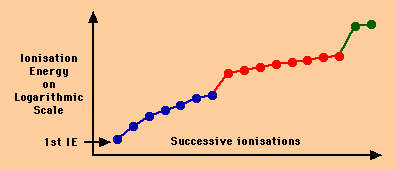
- The first ionisation energy is labelled with an arrow.
- The 7 electrons from the outer shell of the chlorine atom (shown in
blue) are the first to be removed.
- The 8 electrons from the next shell of the chlorine atom (shown in
red) are the next to be removed.
- Finally, the two electrons from the inner shell of the chlorine atom
(shown in green) are removed.
Note that in an A level exam you will not necessarily be shown all the
successive ionisation energies of an atom, you may only be shown the first
10 or so, but this should still be enough information to deduce how many
electrons the atom has in its outer shell and therefore to which group
in the Periodic Table it belongs.
Module Index | Topic
Index | Previous Page | Next
Page

