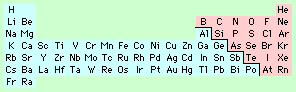
Compounds containing two elements (so called binary compounds) can either have ionic or covalent bonding.
To decide if a binary compound has ionic or covalent bonding, first locate the two elements concerned in the Periodic Table and decide if they are metals (shown in blue) or non-metals (shown in pink).
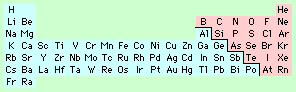
If they are both non-metals (such as carbon and oxygen) they will form a covalent compound (such as carbon dioxide, CO2).
If one is a metal (like sodium) and the other a non-metal (like fluorine), they will form an ionic compound (such as sodium fluoride, NaF).